Cannabis

The cannabis or hemp plant has been known since ancient times and grows in almost all parts of the world, but it’s mainly known as a useful source of fiber for the manufacture of textiles and ropes. In most fiber-producing areas, the plant was not used as a drug. Geographical and climatic factors modify the content of pharmacologically active material in the plant, and only in some regions this content was high enough to discover that the plant, and especially its resin, has important pharmacological actions. Knowledge of these actions appears to have first emerged in the Himalayan region of Central Asia and gradually spread from there to India, Asia Minor, North Africa, and traversed the desert to sub-Saharan Africa and the rest of the African continent.
In India, the plant was used as medicine and in other practices. Its social and religious uses were mainly related to the Durga Puja festival. On some other occasions during the year it was also used in family celebrations, such as marriages and births, to induce a relaxed and social mood and a good appetite. Only the weakest preparations were used: ‘bhang’ (comparable to marijuana) was taken orally, and the slightly stronger ‘ganja’ preparation was smoked, but the more potent preparation, ‘charas’ (known elsewhere as hashish), was not used for these purposes. , the use of charas was not socially approved for any purpose, and its devotees were considered ‘bad characters’ or marginalized.
Cannabis was also part of the therapeutic arsenal of traditional Indian medicine, and many of the uses were similar to those currently recommended in our own society. Among its stated benefits are sedative, relaxing, anxiolytic and anticonvulsant actions, all of which also made it useful in the treatment of alcohol and abstinence of opiates,analgesia, stimulation of appetite, antipyretic and antibacterial effects, and relief of diarrhea.
The introduction of the effects of the cannabis drug in Europe in the 19th century followed different routes for medical and non-medical uses. In France, general interest focused on the non-medical application of psychoactive effects, while in England the interest was primarily medical. During the Napoleonic invasion of Egypt in 1798, De Sacy and Rouyer, two French scholars who accompanied the army, described the plant and the practice and effects of smoking, and collected samples of the material to bring to France for further study. The famous French psychiatrist Moreau de Tours made further observations of its effects on the mood during his travels through North Africa in the 1830s. He later described in detail the mental effects of high doses of hashish, and advanced the hypothesis that dreams, insanity, and drug intoxication involve similar mechanisms. He proposed the use of hashish to produce a “model psychosis” for scientific study, a century before this concept was proposed in North America in relation to the hallucinogens lysergic acid diethylamide and mescaline. In Paris, the ‘Club des Haschichins’ flourished in the 1850s, with members such as the poets and authors Baudelaire, Gautier and Dumas. They served as subjects for Moreau’s experiments and popularized hashish in his writings as a route to aesthetic self-actualization, as did Ginsberg and others in the United States a century later.
In the United Kingdom, on the other hand, the medical and scientific writings of O’Shaughnessy, a British doctor working in India as a professor of Chemistry and Medical Matter in Calcutta, sparked interest in cannabis and observed the use of cannabis in India in traditional medicine, for the treatment of spastic and convulsive disorders such as ‘hydrophobia’ (rabies), tetanus, cholera and delir-ium tremens. He sent supplies of the material to a pharmaceutical company in London for analysis and clinical trials. Cannabis extracts were adopted in the British Pharmacopoeia and later in the American Pharmacopoeia, and were widely used in the English-speaking world as sedative, hypnotic, and anticonvulsant agents in the late 19th and early 20th centuries.
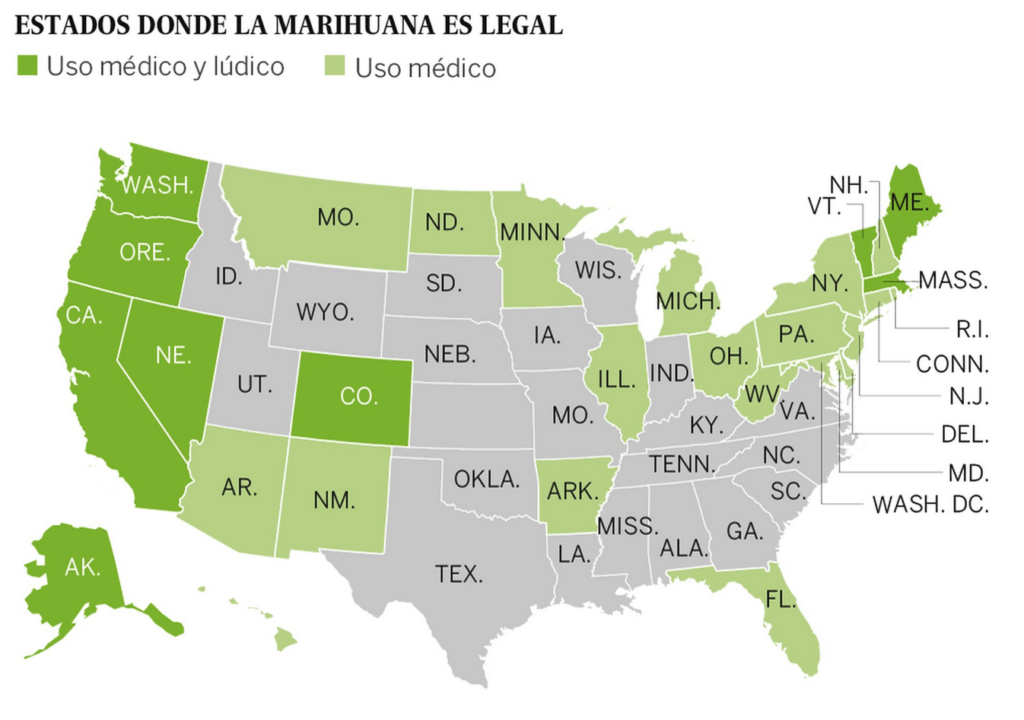
However, when cannabis was removed from the British Pharmacopoeia in 1932 and from the American Pharmacopoeia in 1941, its clinical use had virtually disappeared and its formal banishment sparked little or no protest. One of the reasons for this loss of favor was that the plant material was too variable in composition, its shelf life was too short and unpredictable, and had been increasingly replaced by pure opiates and new, more reliable synthetic drugs invented in the first part. Thus, cannabis would have to be substantially improved as a drug to regain clinical interest.
Ancient and modern chemical studies.
In North Africa the very high lipid solubility of materials responsible for the effects of cannabis on drug use was known, where a common practice was to heat the leaves and the upper part of the plant in a mixture of butter and water. The active drugs were concentrated in the butter phase and, as the mixture cooled, the butter could be separated from the water and used in preparations to be taken by mouth to produce the desired effects. In 1857, the Brother Brothers of Edinburgh prepared a non-alkaloid fraction with a high level of pharmacological activity, and the alcoholic extracts or the dry residues obtained from them were later standardized for their biological activity, forming the basis of pharmacopoeial preparations. In 1899 Wood, Spivey, and Easterfield attempted to isolate the active agents from such preparations, but their “cannabinol” had very little pharmacological activity and turned out to be a mixture rather than a single compound.
It was not until the 1930s and 1940s that Todd et.al In the UK and Adams et. al. In the United States they isolated pure cannabidiol and various tetrahydrocannabinoles (THC), and showed that the latter were responsible for the psychoactive effects. Of the numerous chemical compounds isolated from cannabis, only three have the typical psychoactive effects for which cannabis is used non-medically: ∆9-THC, ∆8-THC, and (very weakly) cannabinol. A fourth natural cannabinoid, cannabidiol, has other types of pharmacological activity but is not psychoactive.
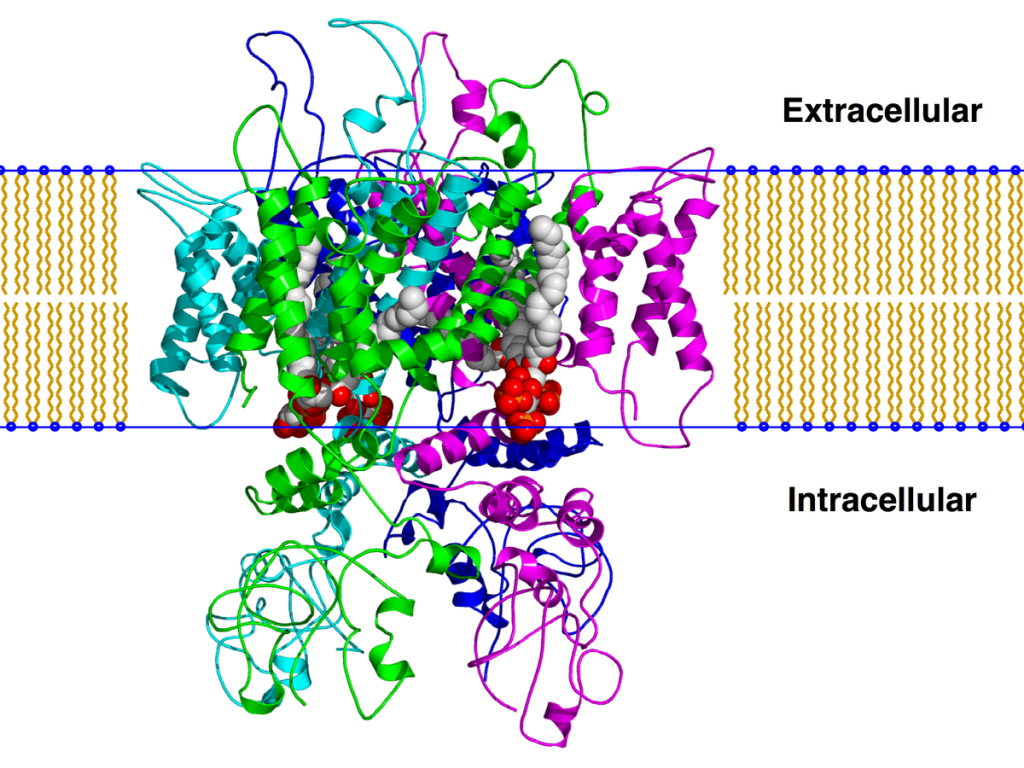
Finally, Mechoulam et.al. In Israel, and Claussen and Korte in Germany achieved the complete synthesis of the pure compounds, established their molecular structures and developed the study of their structure-activity relationships. This work led to the synthesis of new cannabinoid derivatives and analogs that do not exist in nature. Armed with these pure and powerful chemicals, Devane et.al. Identified specific binding sites (cannabinoid receptors) in the brain, and demonstrated the receptor binding affinities of the different compounds parallel to their respective potencies of biological activity.
Because cannabinoids themselves do not exist in the brain, the existence of the receptors implies that other endogenous materials in the brain normally bind to them. Devane et.al. later reported the isolation of anandamide (arachidonyl ethanolamine), a lipid material related to prostaglandins, which is formed locally in the brain and binds to receptors, exerting actions similar to those of cannabinoids but less potent. Arachidonyl-glycerol and several other similar materials have been subsequently identified.
Cannabinoid receptors were found to be of at least two different types, CB1 receptors are mainly present in various parts of the brain (cerebral cortex, cerebellum, basal ganglia, limbic system, hypothalamus, hippocampus) and CB2 receptors present exclusively in peripheral tissues such as the immune system, bone marrow, lung, pancreas, and smooth muscle. Both types of receptors are linked to the inhibitory G protein, through which they act to inhibit cyclase activity, preventing theactivation of various calcium ion channels in the cell membrane, while increasing the entry of potassium ions. Functional results vary on different types of neurons. Inhibitory neurons are activated, with increased release of GABA, while motor neurons, cellular excitability, andneurotransmitter release decrease. The isolation of the different types ofreceptors has allowed the development of fully synthetic compounds with high selective affinity for one type or another, some acting as agonists and others as antagonists. The availability of these receptor-specific ligands has allowed rapid advances in the analysis of the cellular mechanisms underlying the various pharmacological effects of cannabinoids.
When examining a cannabis flower, you will notice a complex knot of different parts: the fiery orange hairs, the sugary crystals, the thick bumps wrapped in small leaves. But what exactly are these formations and what functions do they fulfill?




This short guide to the anatomy of cannabis is intended to familiarize you with the plant in its full form.
Male and female plants


Cannabis plants can be male, female, or both (hermaphrodite).
Female plants produce the large resin-secreting flowers that are trimmedinto round or pointed buds, while males produce smaller sacks of pollen near the base of the leaves. Male plants pollinate females to start seed production, but the powerful flowers we consume come from female seedless plants, called “sin semilla”, which produce large buds rich in seedless cannabinoids.
Rare hermaphrodite plants contain male and female sex organs that allow the plant to pollinate itself during flowering. This self-pollination is generally considered a nuisance among growers, as it spoils seedless seedless plants and transmits hermaphrodite genes.
Growers can ensure the sex of their plants by growing clones or genetically identical clippings of an original strain. Feminized seeds are also available through a special breeding process.
The cannabis plant is made up of several structures, many of which can be found in any common flowering species. Cannabis grows on long, slender stems with its large, iconic fan leaves that extend from areas called nodes. Cannabis really begins to stand out in its flowers, where unique and intricate formations occur.
Tail


A tail refers to a group of shoots that grow close together. While the smallest tails occur along the budding sites of the lower branches, the main tail (sometimes called the apical shoot) forms at the top of the plant.
Stigma and pistil


The pistil contains the reproductive parts of a flower, and the vibrant, hair-like filaments are called stigmas. Stigmas are used to collect pollen from males. The stigmas of the pistil begin with a white coloration and progressively darken to yellow, orange, red and brown throughout the maturation of the plant. They play an important role in reproduction, but stigmas add very little to the potency and flavor of the flower.
Bracts and calyx
A bract is what encapsulates the female’s reproductive parts. They appear as green teardrop “leaves”, and are heavily covered with resin glands that produce the highest concentration of cannabinoids of all parts of the plant. Enclosed by these bracts and imperceptible to the naked eye, the calyx refers to a translucent layer on the ovule at the base of a flower.
Trichome
Despite its diminutive size, the crystal resin blanket is hard to miss in a cannabis bud. This resin (or “kief” when dry) is secreted through translucent mushroom glands in the leaves, stems, and calyces. Trichomes were originally developed to protect the plant against predators and the elements. These clear bulbous balloons ooze aromaticoils called terpenes, as well as therapeutic cannabinoids like THC and CBD. The basis for the production of hashish depends on these trichomes and their powerful sugar-like resin.
The words “indica” and “sativa” were introduced in the 18th century to describe different species of cannabis: Cannabis sativa and Cannabis indica. The term sativa describes the hemp plants found in Europe and western Eurasia, where it was grown for its fiber and seeds. Cannabis indica refers to the psychoactive varieties discovered in India, where it was harvested for its seeds, fiber and hashish production.
Although the cannabis varieties we consume come largely from Cannabis indica, both terms are used, even mistakenly, to organize the thousands of strains that are on the market today.
This is how the terms have changed since their first botanical definitions:
- • Today, “sativa” refers to tall, narrow-leaf cannabis varieties, which are believed to induce energizing effects. However, these narrow leaf drug varieties were originally Cannabis indica ssp. Indicates
- “Indica” has come to describe robust broad-leaved plants, believed to produce sedative effects. These broadleaf drug varieties (BLD) are technically Cannabis indica ssp. Afghan
- • What we call “hemp” refers to non-intoxicating industrial varieties harvested primarily for fiber, seed and CBD. However, this was originally called Cannabis sativa.
With the massive commercialization of cannabis, the taxonomic distinctions between cannabis species and subspecies became upside down and calcified. But now we know that a strain has more than its indica, sativa or hybrid designation.
Hybrid cannabis
The hybrid strains are bred from descendant indica and sativa plants. Due to the long history of cannabis strain crossings, many of them historically made underground to evade authorities, strains that have purely indica or sativa lineages are truly rare. Most of the strains called “indica” or “sativa” are, in fact, hybrids, with inherited genetics from both subspecies.


Cannabinoids and TerpenesBoth THC and CBD are considered the best known cannabinoids. There are hundreds of cannabinoids in cannabis and more than 65 cannabinoids have already been classified. I In total, there are over 480 natural components within the cannabis plant.


THC (tetrahydrocannabinol) is the part of the cannabis plant that produces a mental and bodily high. It has mind altering effects. THC works by binding to the brain’s natural cannabinoid receptors, which create a feeling of euphoria. Many people consume THC through oils, capsules, flowers, and food.
CBD is very different. CBD does not have a disruptive effect and is used to treat many different ailments. CBD comes from hemp and cannabis plants. Products are created by extracting CBD oils from the plant and turning them into a gel, jelly beans, oil, or supplement.
Generally speaking, CBD and THC help treat medical conditions and disorders. Large doses of THC can have some negative side effects, while large intakes of CBD are generally safe, as it is not a mental disorder.
CBD can help treat your furry loved one’s anxiety and help them calm down when they are stressed. You can give them CBD by getting treats, oils, and water drops.
According to studies, any CBD that is safe for human consumption should also be safe for pets and children. However, only a limited number of veterinarians actually recommend CBD for dogs, as there are a limited number of studies. More study needs to be done to see if it can work with both cats and dogs.
Cannabis Terpenes


Cannabinoids and terpenes have many differences. Terpenes are natural essential oils that control how things smell and taste. You have been experiencing them all your life!
There are at least 20,000 different terpenes, and the cannabis plant contains more than 100 of them.
Both Indica and Sativa plants have terpenes. These terpenes help determine which plant or strain you have. The types of terpenes within plants can even tell you if it’s a Sativa, Indica, or hybrid.
One of the most important terpenes to know is myrcene. This terpene has anti-inflammatory and sedative properties. Any strain that has more than 0.5% myrcene is considered Indica, due to the sedative effect of myrcene when combined with THC.
Sativa plants contain less myrcene and sometimes high levels of pinene. Pinene also has anti-inflammatory properties, but it also increases your mood.
Humulene terpene is good for people who want to decrease their appetite and benefit from its antibacterial properties.
Caryophyllene terpene can make your marijuana have a pungent or pungent aroma. This terpene can benefit people who experience anxiety, depression, and inflammation.
Linalool terpene is best for those who want a relaxing and stress-relief experience. This terpene can actually help reduce anxiety about THC.
As mentioned earlier, terpenes control how we perceive taste and smell. Many times, people select their favorite variety for the taste or smell of the flower. If you know which terpenes are dominant in that strain, you can easily find other strains with the same profile.